In the evolving biosimilar landscape, patent challenges remain a critical strategy to clear the path for market entry. Recent reforms at the Patent Trial and Appeal Board (PTAB) under Director John Squires have significantly altered the inter partes reviews (IPRs) landscape, leading to a steep decline in institution rates. While this shift may initially appear as a hurdle for biosimilar developers seeking to challenge blocking patents, it opens the door to underutilized alternatives like ex parte reexaminations and it increases the criticality of filing post-grant review (PGR) petitions when the opportunity arises (i.e., within 9-months of patent grant). Here we explore these changes, their impact on biosimilars specifically, and why reexams could emerge as a strategic tool in biosimilars manufacturers’ toolboxes.
PTAB Reforms and the Decline in IPR Institutions
Director Squires implemented procedural changes aimed at enhancing efficiency of PTAB proceedings. These changes include centralization of institution decisions under his direct oversight (effective October 2025), proposed rules addressing serial petitions, and real-party-in-interest requirements [1]. These reforms have dramatically decreased IPR institution rates initially. IPR filings declined to 60.4% of total PTAB petitions in 2025 (down from 73% in 2024), procedural denials surged 630% (i.e., to a record 607), and institution rates plummeted from 65-82% in the first quarter to 15-34% by the third quarter [2]. See FIG. 1 (illustrating drop in instituted IPRs); see also FIG. 2 (illustrating slight increase in instituted PGRs). A full review of fiscal year 2025 trial statistics at the PTAB can be found on our PTAB Law Blog.
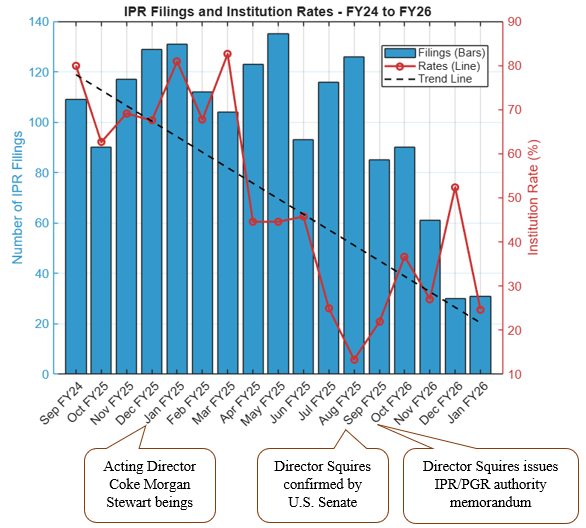
FIG. 1: Demonstration of IPR filings and institution rates from September 2024 through January 2026. Data for January 2026 were collected through January 25, 2026. The blue bars depict the number of IPR filings for each month; the red line depicts the institution rate (%), where the institution rate is calculated from the number of petitions instituted divided by the total number of decisions made (instituted and denied), excluding settlements, terminations, pending petitions, etc. The trend line shows a downward trend of IPR institution rates. Appended notes depict important dates. Data collected January 25, 2026, and are available at https://data.uspto.gov/ptab/trials/proceedings.
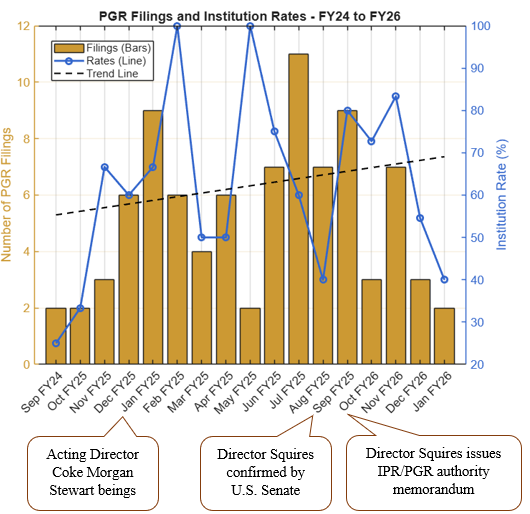
FIG. 2: Demonstration of PGR filings and institution rates from September 2024 through January 2026. Data for January 2026 were collected through January 25, 2026. The yellow bars depict the number of PGR filings for each month; the blue line depicts the institution rate (%), where the institution rate is calculated from the number of petitions instituted divided by the total number of decisions made (instituted and denied), excluding pending petitions. The trend line shows a slightly upward trend of PGR institution rates. Appended notes depict important dates. Data collected January 25, 2026, and are available at https://data.uspto.gov/ptab/trials/proceedings.
Historically, IPRs have been a favored tool for biosimilar applicants under the Biologics Price Competition and Innovation Act (BPCIA), allowing challengers to invalidate key patents efficiently [3, 5]. However, with stricter scrutiny, denials have increased, which may potentially prolong patent disputes and delay biosimilar launches. Recent analysis suggests that IPR institution rates may be climbing off the recent lows, but the rate remains well below the historical average of roughly 67% [7].
Ex Parte Reexaminations: An Underutilized Avenue for Biosimilar Challengers
Amid these PTAB changes, ex parte reexaminations stand out as a promising, yet underutilized, alternative to IPRs. Unlike IPRs, which involve adversarial proceedings (albeit with historically high invalidation rates averaging around 60-70%), ex parte reexams are conducted between the patent owner and an examiner, after the reexamination request is filed by a requester. While the USPTO grants 95% of ex parte reexam requests (vs. 63% institution for IPRs), reexams have lower claim cancellation rates (only 14%) [4]. Approximately 62% of patents that undergo reexamination end up with modified or canceled claims [4]. Filings for ex parte reexams surged 66.1% to 726 in 2025, likely driven by the IPR institution declines [2]. See FIG. 3:
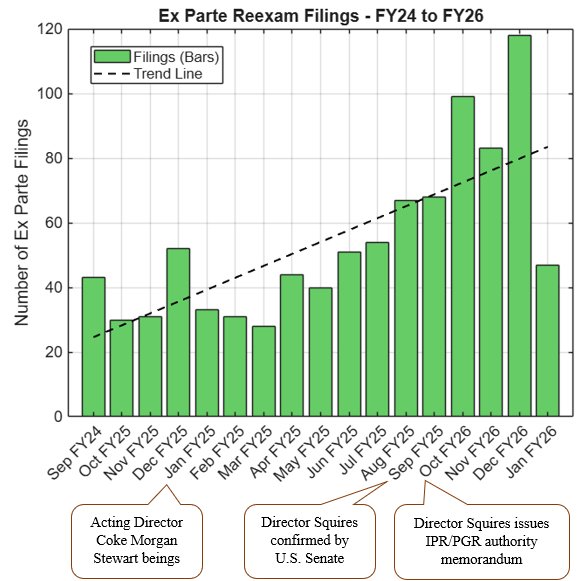
FIG. 3: Demonstration of ex parte reexamination filings from September 2024 through January 2026. Data for January 2026 were collected through January 25, 2026. The green bars depict the number of ex parte reexamination filings for each month. The trend line shows an upward trend of ex parte reexamination filings. Appended notes depict important dates. Data collected January 25, 2026, and are available at https://portal.unifiedpatents.com/exparte/analytics.
For biosimilars developers, an ex parte reexam provides several advantages. The process is cost-effective (i.e., filing fees are around $6,000 vs. $40,000+ for IPRs), anonymous (i.e., no real-party-of-interest disclosure), and flexible, which allows challenges based on printed publications without estoppel risks in subsequent litigation [4]. In biologic patent disputes, where complex formulations and methods are common, ex parte reexams can target narrow invalidity grounds effectively. Another key benefit is that challenges based on double patenting are allowed, which can be critical for challenging patents that extend pharmaceutical exclusivity. However, drawbacks include inability to assert enablement (35 U.S.C. § 112) and eligibility (35 U.S.C. § 101) challenges, and no direct interaction with the examiner after filing the request. Meanwhile, patentees can conduct examiner interviews, submit expert declarations, amend claims, and argue iteratively.
Proposed ‘one-and-done’ rules could bar subsequent PTAB challenges if a patent is upheld in reexam or court, potentially bulletproofing it from further challenges [6]. We are closely monitoring developments in regard to this proposal and will continue to provide updates.
The uptick of ex parte reexams could represent an adaptation to patent challenge strategy. Biosimilar applicants can use them strategically to cancel key claims of blocking patents while minimizing costs and avoiding the uncertainty around IPR institutions. This underutilized tool provides an alternative avenue for challengers to clear patent thickets blocking biosimilar entry.
Impact on the Biosimilars Patent Landscape
Over the years, the biosimilars market has seen robust growth, with PTAB challenges playing a pivotal role in facilitating competition. For example, in pharmaceutical disputes, IPRs historically achieved high institution rates (around 73%) and contributed to settlements that enabled earlier market entry [3]. Under the new regime, lower IPR institution rates shift most patent challenges to district courts, rather than faster and cheaper resolutions at the PTAB, unless PGRs and ex parte reexaminations can fill the void. District court litigations can favor patentees, where patents enjoy a presumption of validity and the standard for proving invalidity is clear and convincing evidence as opposed to the PTAB’s lower standard of a preponderance of the evidence and no presumption of validity.
For biosimilar developers, and for challengers in general, the procedural changes signal a directive from the USPTO to severely limit duplicative or late challenges, favoring recently granted patents. Medical and pharmaceutical petitions, including those for biologics, remained relatively steady at 12.7% of PTAB activity in 2025, indicating sustained interest despite the uncertainty [2]. Challengers may need to adapt strategies, such as focusing on stronger prior art (e.g., airtight, single grounds) to avoid denial or considering the procedural factors likely to impact institution, such as parallel litigation or real-party-in-interest disclosures.
Looking Ahead
While declining IPR institutions may reshape biosimilar strategies, the situation is likely to lead to a rise in ex parte reexaminations as well as PGRs (when available). Ex parte reexaminations offer an efficient, low-risk alternative that biosimilar developers should consider, particularly when double patenting-based challenges are available. These proceedings remain viable under the new regime, and provide alternative pathways to clear patents blocking biosimilar entry.
References
1. USPTO Proposes New Institution Rules and Director Takes Over Merits-Based Institution Decisions, Morgan Lewis. (available at https://www.morganlewis.com/pubs/2025/10/uspto-proposes-new-institution-rules-and-director-takes-over-merits-based-institution-decisions).
2. Patent Dispute Report: 2025 in Review, Unified Patents. (available at https://www.unifiedpatents.com/insights/2026/1/13/patent-dispute-report-2025-in-review).
3. Biosimilar Patent Dance: Leveraging PTAB Challenges for Strategic Advantage, Drug Patent Watch. (available at https://www.drugpatentwatch.com/blog/biosimilar-patent-dance-leveraging-ptab-challenges-for-strategic-advantage/?srsltid=AfmBOopbJSNMW1vtWQXpBWyZmCaOvOLuVZMIDMiZDg53ErL0y3ZRinAs).
4. Ex Parte Reexamination Process, Adibi IP. (available at https://adibiip.com/ex-parte-reexamination-process/).
5. Cracking the Biosimilar Code: A Deep Dive into Effective IP Strategies, Drug Patent Watch. (available at https://www.drugpatentwatch.com/blog/cracking-the-biosimilar-code-a-deep-dive-into-effective-ip-strategies).
6. The Slow Death of AIA Trials: A Look into the USPTO’s ‘One-and-Done’ Rule Package, IPWatchdog. (available at https://ipwatchdog.com/2025/12/09/slow-death-aia-trials-one-and-done/).
7. The Thaw Begins?: What’s Driving IPR Institutions Under Director Squires, Patently-O. (available at https://patentlyo.com/patent/2026/01/driving-institutions-director.html).
Disclaimer: The information contained in this posting does not, and is not intended to, constitute legal advice or express any opinion to be relied upon legally, for investment purposes or otherwise. If you would like to obtain legal advice relating to the subject matter addressed in this posting, please consult with us or your attorney. The information in this post is also based upon publicly available information, presents opinions, and does not represent in any way whatsoever the opinions or official positions of the entities or individuals referenced herein.
